Auditory Safety & Efficacy Services from CBSET & Cilcare
CBSET and hearing specialists, Cilcare, have partnered to offer a broad range of cutting-edge preclinical auditory safety and efficacy services to assess drugs, medical devices, and gene and cell-based therapies for auditory functions in a GLP-compliant environment.
6 Years of Partnership Milestones
Since partnering with Cilcare, we have conducted numerous GLP and non-GLP safety and efficacy studies on a wide range of hearing therapies.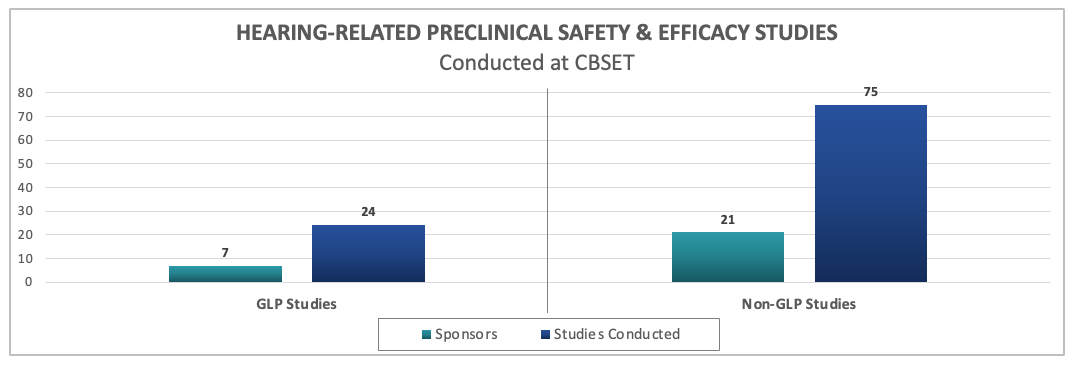
One of these therapies, designed to provide durable restoration of hearing to individuals born with profound hearing loss due to a genetic mutation, recently gained FDA approval for clinical trials.
Extensive History in Otology Research
With expertise in both GLP and non-GLP otology research, we provide a broad range of in vivo models, enabling studies from proof-of-concept and non-GLP programs through fully GLP-compliant safety assessments. Inner ear and systemic pharmacokinetics, ototoxicity, and safety and efficacy studies may be conducted in a variety of preclinical species and models including device-mediated therapy (such as cochlear implants and implantable drug delivery systems), biologics and therapeutic proteins, small molecules, cell products, or gene therapy vectors, all in accordance with best practices and applicable regulatory guidelines.
A Broad Range of Technical Capabilities for Auditory Therapy Assessment
Our technical capabilities include in-life assessment with high-throughput electrophysiological measurements and post-mortem assessments via immunohistochemistry, histopathology, and hair cell and ribbon synapse quantification. In addition, our otic research scientists and surgeons can provide method development support to adapt or replicate challenging technical procedures including cochleostomy, canalostomy, and round window injections targeting different sites of interest.
- Expertise in auditory assessment
- ABR (Auditory Brainstem Response) measurements
- Otoscopic evaluation
- Tympanometry
- Cytocochleogram
- Ribbon synapses counting
- Histopathology and immunohistochemistry
- Imaging
- Cochlear explants
- Animal models
- Drug-induced ototoxicity
- Otitis media
- Animal species
- Rodents
- Guinea pigs
- Chinchilla
- Swine
- Sheep
- Routes of delivery
- Transtympanic
- Round window
- Intracochlear
- Intrabullar
- Samplings
- Perilymph & cochlea
- Tympanic bulla
- CSF and other tissues
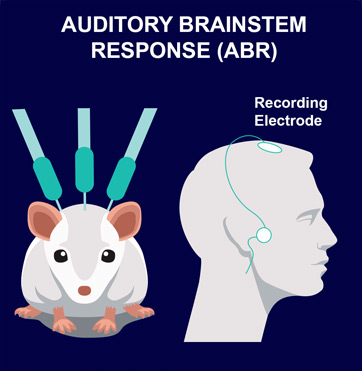
ABR are electric potentials recorded from scalp electrodes. This non-invasive method allows determination of auditory response thresholds. It is translational between rodents and humans, and is commonly used for screening newborns.
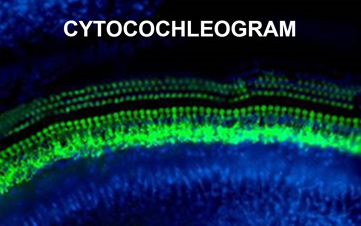
The cytocochlelogram allows histopathological assessment of ototoxic damage on inner hair cell and outer hair cell of the cochlea, with anatomical localization corresponding to predicable patterns of functional deficits.
CBSET Partner Cilcare wins French-American Chamber of Commerce of New England (FACCNE) Award in the "Women in Business" Category